December 2023
A case of inter-pacemaker crosstalk
Daniel Hunnybun Highly Specialised Cardiac Physiologist, Northern General, Sheffield, UK
Disclosure: The author has no conflict of interests to declare.
Background
A patient with adult congenital heart disease presented to pacing clinic with palpitations. Notably the patient had 2 pacemakers in situ.
The patient had a epicardial dual chamber ELA medical pacemaker implanted following their TCPC surgery in 2004 due to sinus arrest. In 2022 the patient had an endocardial single chamber atrial Medtronic device implanted following RV lead failure and the inability to programme around far field R wave oversensing. Following the transvenous device implant the epicardial and endocardial devices were programmed to AAI 30bpm and AAIR 70bpm respectively.
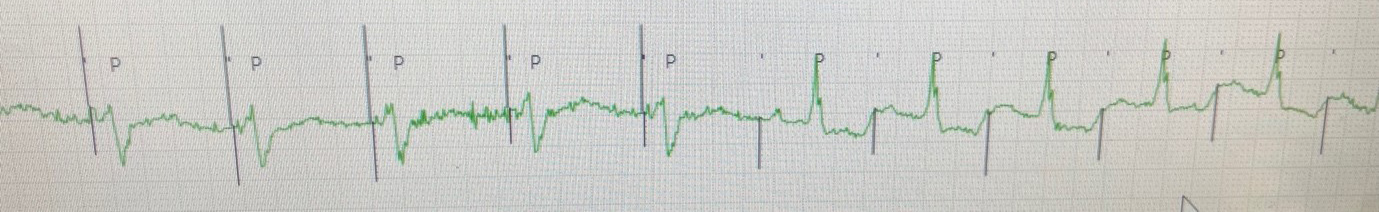
On presentation in pacing clinic the following EGMs/ECGs were recorded (Figure 1.).
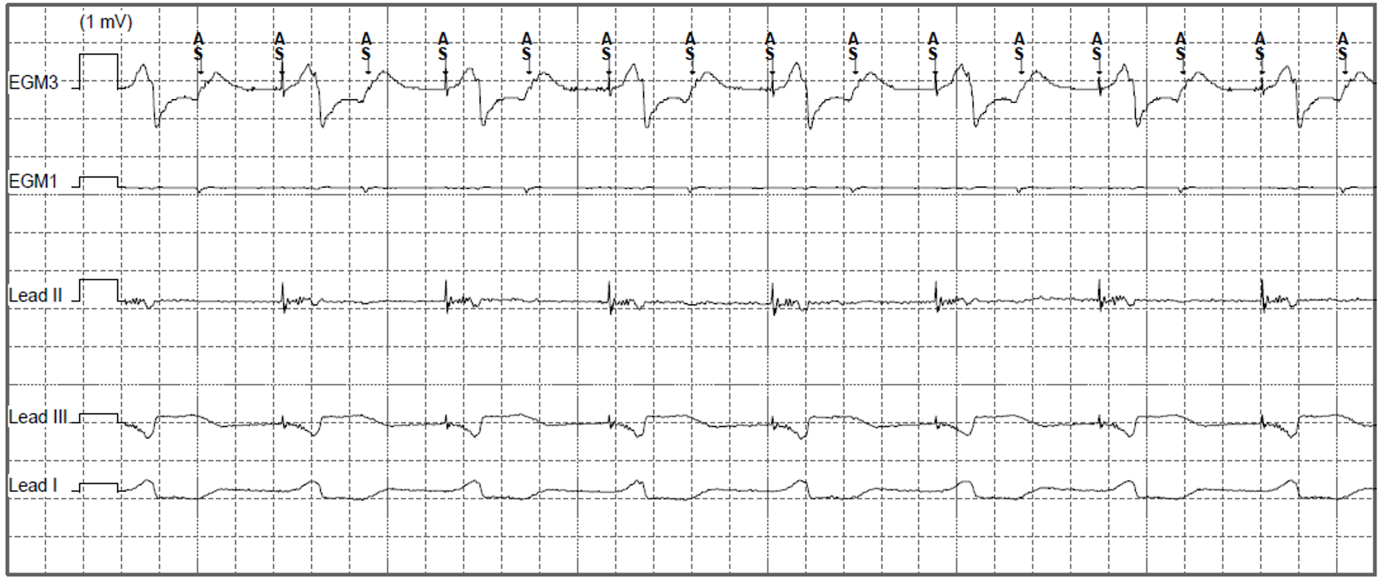
Figure 1. Presenting EGM in pacing clinic (EGM3: Can-Aring, EGM1: Atip-Aring).
The epicardial device was found to be at ERI and had consequently mode switched to VVI 70bpm with unipolar pacing (5V@1ms). As well as VA conduction, inter-pacemaker crosstalk can explain the higher sensed atrial rate seen in Figure 1 (Bardia et al. 2022). The epicardial device was reprogrammed to VVI 30bpm with cessation of ventricular pacing. The patient was then admitted for their epicardial device to be explanted.
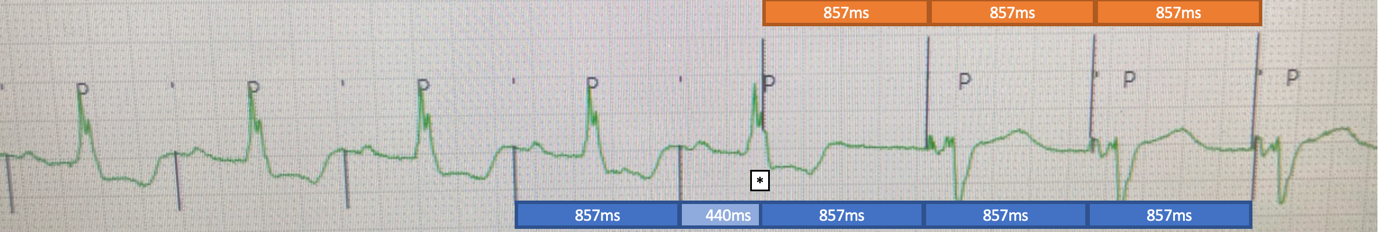
The following morning the patient’s symptoms returned with the telemetry changes seen in Figure 2.

Figure 2. Telemetry changes at time of symptom onset.
QUESTION
What is the most appropriate programming change to resolve the patient’s ongoing symptoms?
Disclaimer: The British Heart Rhythm Society (BHRS) collates submissions for the ECG/EGM challenge on this website. These submissions, along with any accompanying answers, are provided by external contributors and are published for informational purposes only. BHRS does not endorse, guarantee, or warrant the accuracy, completeness, or reliability of any submissions or answers provided.