Answer
PVC with Pseudopseudofusion
Explanation/Discussion
There are 3 primary aspects to consider when interpreting this EGM.
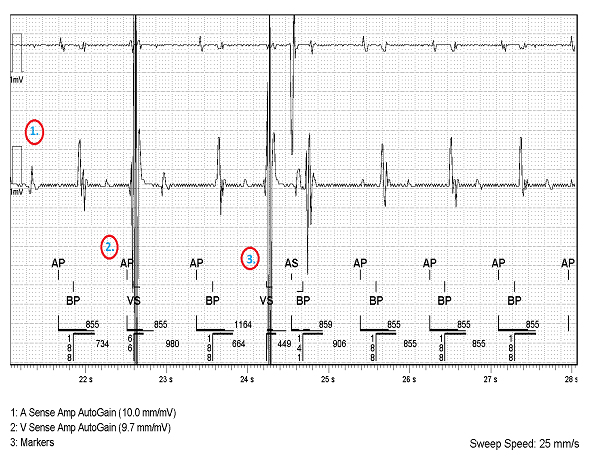
- The V sense amplitude AutoGain has been ramped up to 9.7mm/mV hence displaying signals at very high amplitude.
- This VS signal is indeed a PVC. In this Abbott CRT-D the V blanking period is 52ms and the ventricular safety standby is 52ms to 65ms. As the PVC is sensed 66ms after the AP is delivered, the PVC falls just 1ms outside of the ventricular safety standby. The PVC therefore occurred during the AV delay and the next marker is an AP delivered after the A-A timer ends.
- The overlap between the AP and the intrinsic PVC means that the PVC is a pseudopseudo fusion beat.
In reference to cardiac implantable electronic devices:
- A fusion beat can be defined as a combined depolarisation of the cardiac tissue from intrinsic depolarisation and the pacing pulse. The QRS complex of a fusion beat on a surface ECG has a unique morphology midway between the intrinsic and paced morphology.
- A pseudofusion beat differs from a fusion beat in that the majority of depolarisation of the cardiac tissue is by intrinsic depolarisation and the ventricular pacing pulse captures little, or no, cardiac tissue. The reduced depolarisation contribution of the pacing output is reflected in the surface ECG which is subsequently similar to that of the intrinsic morphology. It is possible to have pseudofusion with a PVC if the VP captures little or no cardiac tissue and therefore does not distort the PVC morphology on the surface ECG.
- A pseudopseudo fusion beat occurs when a spontaneous ventricular signal (commonly a PVC or conducted R wave) coincides with an atrial pacing pulse. The surface ECG morphology reflects the intrinsic activity as is not affected by the pacing stimulus, and therefore it appears on an ECG as pseudofusion, however it is a “fake” pseudofusion hence labelled pseudopseudofusion.
- A PVC is again seen here however this time V to A conduction is seen. The V to A conduction time is 310ms and therefore falls outside of the programmed 250ms PVARP interval, consequently the retrograde conduction is tracked forming 1 beat of a pacemaker mediated tachycardia (PMT). To prevent such inappropriate tracking from occurring in the future the PVARP was extended.
Whilst having no severe clinical impact in this case, there have been reported case studies of device-initiated arrhythmia caused as a direct result of pseudopseudo fusion. An example of such a case is when a PVC falls into the post atrial ventricular blanking (PAVB) period and is therefore not sensed. As a result, a ventricular pace is delivered at the end of the programmed AV delay which coincides with the vulnerable period of the cardiac cycle resulting in the initiation of polymorphic VT/ VF (Do et al., 2021).
It is important to acknowledge that the reported PVC burden in the device counters was underestimated as a result of pseudopseudo fusion events, such as that seen in the trace above, as the PVCs occurring during the AVD are not labelled by the device as PVCs. Whilst the short AV delays seen on the AV conduction histogram can give an indication of the PVC burden, this patient was referred for a 24-hour holter to accurately assess the true PVC burden. Subsequently in order to reduce the risk of device-initiated arrhythmia in this case, the patient was referred for a medication review with the aim of reducing the PVC burden.
This case exemplifies a modern issue facing healthcare providers globally. The increased use of smart watch devices means that healthcare services will inevitably face an increased burden of work evoked by false-positive smart watch alerts. Many smart watches use photoplethysmography (PPG) technology which directly correlates the change in the blood volume in the peripheral circulation to heart rate. It has been reported that this method of heart rate assessment cannot accurately account for PVCs as the reduced stroke volume, caused by reduced diastolic filling in PVCs, resultingly reduces the blood flow in the peripheral circulation beyond that detectable by PPG and hence the heart rate may be significantly underestimated (Kovoor and Thiagalingam, 2021). It is therefore highly likely that the low heart rate smart watch alert received by this patient was inaccurate, resulting from the under sensing of frequent PVCs.
Many thanks to Abbott and specific thanks to Paul Doherty for their speedy and comprehensive support during this case.
Do, D.H., Meyer, S., Bradfield, J., Shivkumar, K., Boyle, N.G. and Khakpour, H. (2021). Masked premature ventricular contractions and intradevice interaction causing ventricular fibrillation. HeartRhythm Case Reports, [online] 7(2), pp.69–73.
Kovoor, J.G. and Thiagalingam, A. (2021). Smartwatch-induced cardiology referral due to pulse underdetection with premature ventricular complexes. HeartRhythm Case Reports, 7(9), pp.585–587.



