Answer
Ventricular lead displacement
Explanation
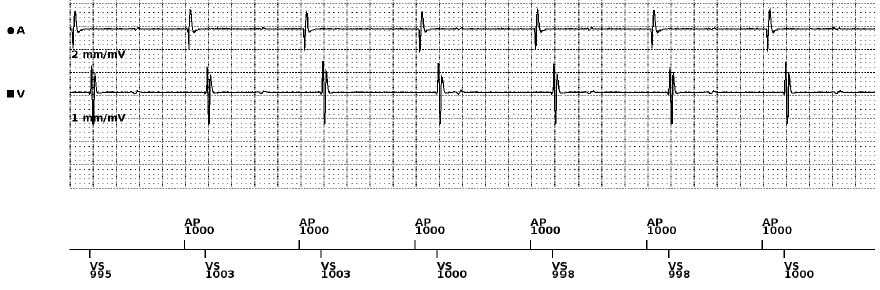
On initial review of our presenting AEGM (Figure 1) we can see an AP marker with local atrial capture. On the VEGM we can see a sharp signal ~160ms post-AP, in line with our VS marker. It would not be unreasonable, given the implant indication to assume correct device function based on this atrial paced rhythm with consistent atrio-ventricular conduction.
However, on closer inspection of the VEGM we can see irregular, low amplitude signals which do not correlate with our marker channel. The signals bare no relation to our VS events and are therefore unlikely to be associated with T wave oversensing. Out of context, and in the absence of symptoms or ECG abnormalities, these additional signals may be ignored.
In this case we have a telemetry strip which shows regular bipolar pacing spikes and an irregular narrow complex ventricular rhythm, warranting further investigation given the patient’s symptoms.
Looking at the bigger picture, we have conflicting information from our intra-cardiac EGM and our telemetry ECG. However, when we applied the ECG from the pacing programmer, we were able to simultaneously review our diagnostics to form an accurate clinical picture.
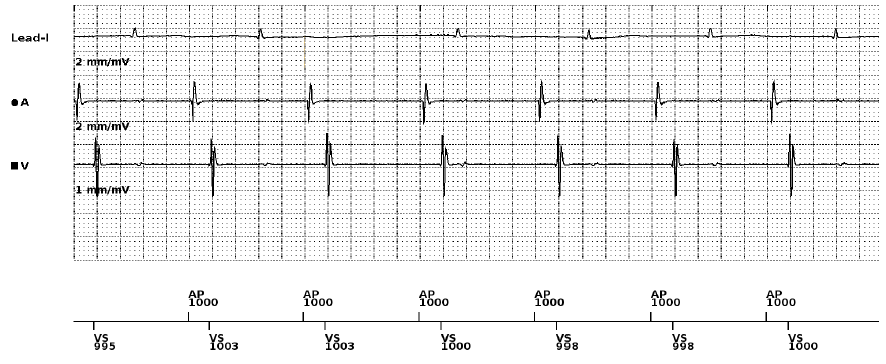
Figure 3 – Presenting EGM and live ECG trace from pacemaker programmer. Channels top to bottom: Lead I ECG, near-field atrial EGM, near-field ventricular EGM and marker channel.
Figure 3 shows how our intra-cardiac EGM directly relates to our surface ECG during simultaneous recording. Now we have put the individual pieces of our puzzle together, it’s clear that our ventricular EGM signals do not line up with our surface QRS complexes.
In this case we present paced P wave oversensing on the VEGM, in the context of latent atrial capture. Each VS signal occurs ~160ms post-AP event, suggesting the two events are related. Our surface QRS complexes are not sensed on our VEGM, therefore our VS signals must be atrial in origin.
Looking back, the patient initially presented with a junctional bradycardia; therefore, we have not been able to comment on AV conduction time. On review of Figure 3, it’s clear this patient has variable AV conduction; reflected in the irregularity of the ventricular rhythm on the surface ECG.
Given the evidence of P wave oversensing and the likelihood of acute lead displacement post-implant, a chest X-ray was ordered to confirm the aetiology of the issue (Figure 4).
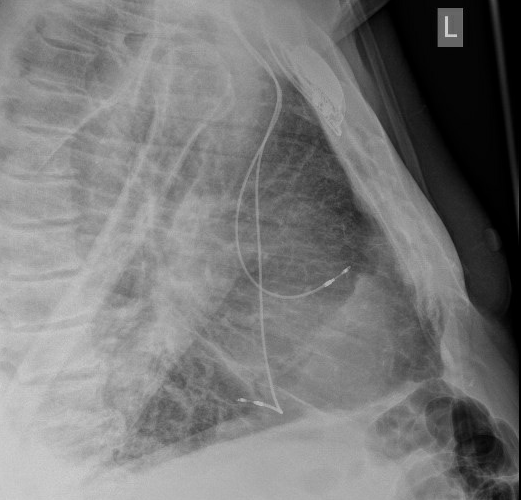
Figure 4 – Lateral chest X-ray performed post-implantation to confirm lead position
Figure 4 shows the right ventricular lead has displaced from the ventricular septum and is freely moving within the right ventricle, close to the tricuspid valve. The proximity of the displaced ventricular lead to the atria explains the paced P wave oversensing.
This patient subsequently underwent a ventricular lead revision and was discharged upon confirmation of satisfactory electrical parameters and absence of symptoms.
This case highlights the importance of a live ECG trace during all routine device checks. Although EGM signals show us what a lead is sensing, this is only relevant if we know where that lead is positioned. Our pacing programmer provides us with a unique opportunity to simultaneously review surface ECG and intra-cardiac EGMs, allowing for a comprehensive review of rate, rhythm, and lead position.
We hope our case highlights the clinical importance of utilising the surface ECG during a device check and the reasons why we should be advocating this as best clinical practice (BHRS, 2022).
References
British Heart Rhythm Society (2022). Clinical Standards and Guidelines for the Follow up of Cardiac Implantable Electronic Devices (CIEDS) For Cardiac Rhythm Management. Available at: https://bhrs.com/wp-content/uploads/2022/06/BHRS-CIED-FU-Standards-June22.pdf. Accessed September 2022.